Project activities & results
The EMRaDi project ended on 31 March 2020 and all the results of the project are presented in this section:
- the final report (online version or PDF version), including a summary of the activities, results and specific recommendations for the different target groups (rare disease patients and their relatives, patient organisations, hospitals and healthcare providers, health insurance funds, policy makers)
- the EMRaDi information factsheet, containing EMRaDi's three main recommendations for political authorities and institutional players
- the information factsheet for rare disease patients and their relatives
- the information factsheet for primary care
- the Declaration of Intent for further cooperation on rare diseases in the Euregio Meuse-Rhine
- the activity reports of the 5 Work Packages (WPs) that have been implemented by the project partners to achieve the project objectives:
WP1 - Evaluation of supply and demand in the field of rare diseases in the Euregio Meuse-Rhine
WP2 - Field analysis of existing rare disease patient pathways
WP4 - Networking, training and exchanges of expertise among health professionals
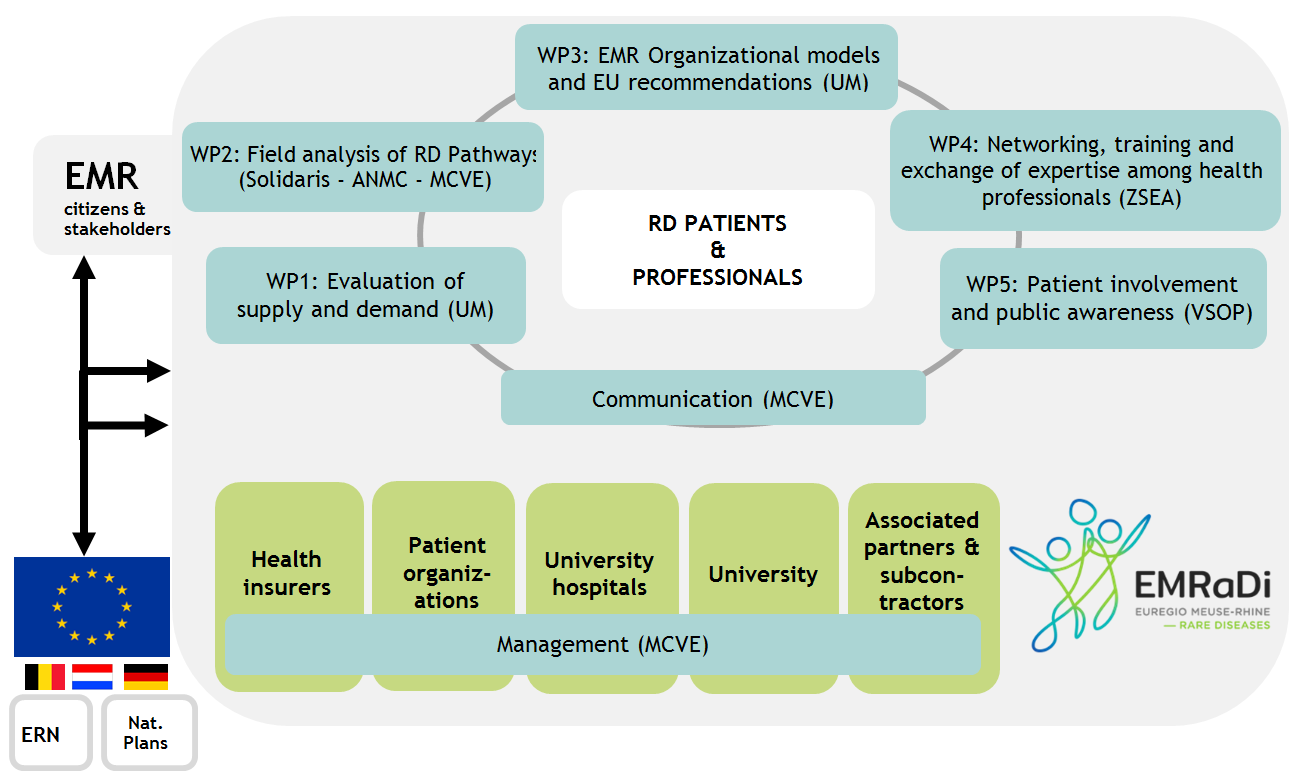
Project management
The Mutualité chrétienne Verviers-Eupen (MCVE), the lead partner of the project, ensured the global coordination and management of the project with the support of the experienced team from its umbrella organization, the Alliance Nationale des Mutualités Chrétiennes (ANMC).
The EMRaDi Steering Committee, which comprises all the partners, met regularly to evaluate the project implementation, share information and coordinate the interconnection of all activities.
Communication
Our communication strategy focuses on the needs of our main target groups:
- Rare disease patients and patients organizations
- Health professionals
- Health insurance providers
- Citizens
- Public authorities
Through our website and other media, we inform our target groups of our events and publications.
Go to the events page to learn more about our communication activities
WP1 - Evaluation of supply and demand in the field of rare diseases in the Euregio Meuse-Rhine
Results
This Work Package, led by Maastricht University, focused on:
- The need and demands of EMR rare disease patients
- Legal and financial aspects of the assistance available for EMR rare disease patients.
The needs assessment was based on a quantitative analysis of 50-60 rare diseases; a report has been drawn up on these rare diseases and the situations of patients suffering from them in the EMR (including legal and financial aspects). In addition, a review of the literature on the needs and demands of rare disease patients and their relatives has been conducted. These analyses took into consideration cross-border care mobility, European Union policies and in particular the national rare disease plans of the three participating countries.
This Work Package aimed to evaluate the supply and demand in the field of rare diseases
WP2 - Field analysis of existing rare disease patient pathways
Results
- Field analysis report of existing RD patient pathways in the EMR
- based on the experience of 104 interviewed participants to uncover the day-to-day reality of RD patients and their relatives
The core concept of a rare disease patient pathway encompasses all the steps from the first symptoms experienced by the patient, along his or her path through the health system towards (hopefully) a fast, successful diagnosis and including every aspect of the care – whether organizational, medical, social, psychological, legal and/or vocational) – to finally ensure the patient the best possible quality of life.
In order to develop organizational models for the management of rare diseases in border regions and to draw up patient-oriented recommendations, this Work Package, led by the Mutualité Chrétienne and Solidaris-UNMS, focused on
- identifying the difficulties patients encounter to
- obtain a diagnosis,
- find a treatment and the necessary non-medical care,
- find health professionals,
- organize their daily lives
- the financial, cultural, linguistic and geographical barriers which may hamper their national and cross-border care.
The project partners met with patients, their relatives and health professionals to collect information through interviews and focus groups.
The outcome of this Work Package is a better evaluation of the needs and of the existing rare disease patient pathways in the Euregio Meuse-Rhine for the eight selected rare diseases.
If you wish to obtain more information about this study, please do not hesitate to contact our team by filling in the subscription form or by sending an email to info@emradi.eu.
WP3 - Development of organizational models for the management of rare diseases in the Euregio Meuse-Rhine
Results
- Patient-oriented models of good practices for the management of rare diseases, with
- generic and specific organisational models in border regions
- recommendations for national and European developments
This Work Package, led by Maastricht University, aimed to identify and develop patient-oriented models of good practice for rare disease patient pathways within and across the different regions of the EMR.
- First, a generic organizational model for the management of rare diseases in border regions has been developed.
- Taking into account the specific situation in the EMR and the three national plans on rare diseases, specific EMR organizational models for the management of selected rare diseases have been developed.
- Finally, based on the experiences in the EMR with the models, recommendations for national and European developments have been formulated.
WP4 - Networking, training and exchanges of expertise among health professionals
Results
- Report summarizing the activities of WP4, with, amongst others,
- the improvement of knowledge on EMR CoE (Centre of Expertise)
- the increase of awareness and training of General Practitioners and resident specialist doctors
- the increase of the cooperation between the EMR partners
- the translational aspects (clinical trials)
- Kabuki as a model project for knowledge dissemination of a new therapy, and the exchange and training of health professionals from the CoE
This Work Package was led by the University Hospital of Aachen and its Centre for Rare Diseases (UKA/ZSEA).
In five activities, the cooperation between the participating university hospitals has been substantially improved and the exchange of patients optimized. This has been done in close cooperation with the other project partners and stakeholders.
On the basis of an exchange of information, common practices for diagnosis and care have been drawn up which have lead to better procedures for the exchange of human material, files and – last but not least and most important – patients.
Exchange programmes, information sessions and communication tools have resulted in:
- a deeper exchange of knowledge between the participating hospitals and
- increased awareness and information among Euregio Meuse-Rhine Health professionals and patients.
We aim for a substantial decrease in the time to diagnosis for patients with the rare disease(s) selected, as this is one of the major problems perceived in the field. Furthermore, the number of patients crossing the border will increase, as will the number of GPs making referrals to CHU Liège, MUMC+ and UKA/ZSEA as specialised centres for rare diseases.
WP5 - Patient involvement and public awareness
Results
- Report including the PSB recommendations, on
- the 3 National Plans for Rare Diseases
- quality documents
- cross-border healthcare
- patient participation in medical research
VSOP (Vereniging Samenwerkende Ouder- en Patiëntenorganisaties voor zeldzame en genetische aandoeningen), the leader of this Work Package, has set up a Patient Sounding Board (PSB) including: Achse (Germany), RaDiOrg (Belgium) and a patient representation from the eight rare diseases selected in the EMRaDi project. Three yearly meetings took place, in addition to regular contacts and teleconferences. In consultation with the PSB, VSOP has:
- Cooperated with, advised and empowered patients (organizations) regarding their role in medical research, health care policy, quality of care, cross-border health care and the patient’s own role in his/her disease management.
- Interacted with the project partners to include the patients perspective in all EMRaDi project activities.
- Interacted with other stakeholders: medical professionals/centres, health insurance providers and policy makers in the Euregio Meuse-Rhine in the field of awareness-raising, exchange of information, best practices and networking. This took place through interaction with partners from the Work Packages 3 and 4 (organizational models and networking professionals).
